Review the Competitive Grant Application Review and Submission SOP for detailed information on the policy, review procedures, application review requirements, and application review criteria.
Key aspects of grant applications at WCM:
- A research grant application is a formal request for financial assistance to a sponsoring agency in support of a proposed project or activity
- Whether to federal or state agencies, private foundations, or industry sponsors, all applications need to be submitted to OSRA for full review and approval prior to submission
- All grant applications require an associated record in the Weill Research Gateway (WRG)
- All submissions require internal approvals by the contact Principal Investigator, the Department Financial Approver or its delegate, and the Department Head Approver or its delegate
- Only certain officials have legal authority to submit proposals or approve submissions on behalf of Weill Cornell Medicine
- Any individual intending to serve as PI on a grant application must be a paid employee and have a primary appointment in a department
- Pre- and Post-Doctoral Fellows and Graduate Students may serve as PI only on grant applications targeted specifically to Fellows and Graduate Students
- Individuals may serve as PIs before their official start date if: 1. A formal acceptance letter signed by all parties is on file with the Department; and 2. The CWID for the person has been activated.
Roles and Responsibilities
Principal investigators are responsible for engaging research administrators as early in the process as possible, and for preparing proposal narratives in sufficient detail to allow adequate review. PIs should collaborate with departmental personnel for the development of project budgets and administrative elements of proposals, such as bio sketches, other support information, research-related assurances, and any documentation of commitments from collaborators at other institutions.
Departmental research administration staff is responsible for following internal policies and review timelines in collaboration with the PI, and for reviewing the sponsor’s guidelines and funding requirements. They are responsible for communicating to investigators the outcomes of OSRA review and for working with the PIs to address comments and requests in a timely manner.
OSRA Grants Specialists are responsible for addressing questions during proposal development, review, and submission process, and for providing guidance to PIs and Departments based on sponsor guidelines, previous experience, and expertise. Specialists must escalate inquiries as needed and in a timely fashion and will provide detailed or limited review feedback, depending on timeline of routing.
Proposal Routing and Timeline
System to System (S2S) records

Non-System to System (non-S2S) records

Frequently Asked Questions
Who can be a PI on proposals submitted at Weill Cornell Medicine?
Any individual intending to serve as PI on a grant application must be a paid employee of Weill Cornell Medicine and have a primary appointment in a Department. Pre- and Post-Doctoral Fellows and Graduate Students may serve as PI only on grant applications targeted specifically to Fellows and Graduate Students.
PIs and/or their DAs must retrieve all relevant submission forms and guidelines for a funding opportunity for which they are eligible and meet program criteria. For an NIH application, PIs must ensure they have a valid and updated eRA Commons username and account. Any questions on eligibility or eRA Commons requests should be directed to the Grants Specialist assigned to the Department’s portfolio.
Do I need to create a WRG record for letters-of-intent or pre-applications? Sometimes they require letters of institutional support and other documents. How can I obtain these?
Letters of Intent and pre-applications are not subject to OSRA’s review and can be prepared and submitted directly to the sponsor by the PI or Department. If the letter of intent or pre-application require institutional signature, the PI or Department staff should contact the respective Grants Specialist to facilitate.
I routed the record, but my Specialist is saying it has not reached OSRA queue for review. What should I do?
Even though the status is automatically updated in WRG, a record will only reach OSRA as an action item once it has been approved by PI and Departmental approvers.
A record routed for pre-review must be approved by the Financial Approver and the Departmental approver before being available to OSRA for review.
A record routed for final review must be approved by the PI, Financial approver and Departmental approver before being available to OSRA for review.
For S2S applications, the “Submission Progress Widget” will show the approval path and pending approvers. Learn more about the widget here.
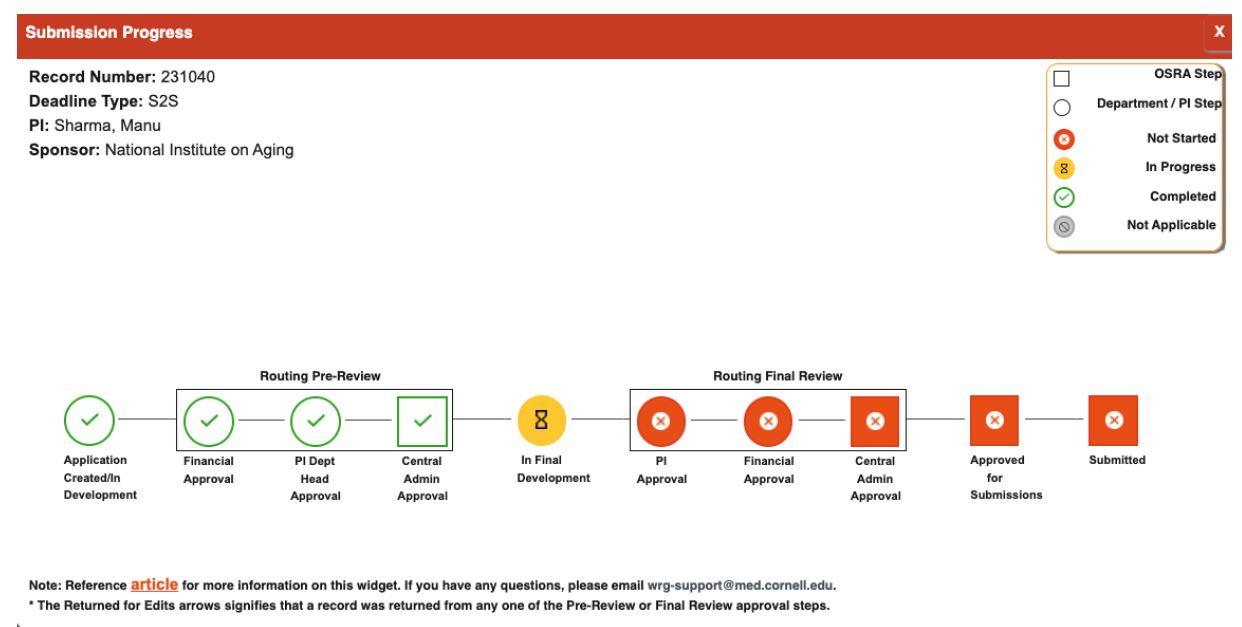
I am looking to apply for an opportunity that is limited to only one application per institution. Can I simply start the record and route for review?
No. Limited submissions must be approved by the Office of the Research Dean. Typically, they will send out calls for submission for internal review for these opportunities, but if it happens for a PI/Department to come across an opportunity that limits the number of submissions by the applicant organization, the Department should contact the Office of the Research Dean expressing interest and providing a general outline of the proposed project
I am working on an NIH resubmission and the system is asking for a Federal Identifier. What is that?
All revisions and resubmissions must include the Institute/Center and serial number of the award must be listed on 4 a. Federal Identifier of the SF424 cover page (as in AB123456 if it is a resubmission of grant 1 R01 AB123456-01)
My proposal has key personnel that will not be included in the budget. How can I add them to the application?
There are many applications in which collaborating personnel will not be committing effort/having salary support. Those are typically mentors/sponsors on fellowships and career development awards, members of advisory boards, and other significant contributors/collaborators. While they need to be listed as key personnel in order for their profile and biosketch to appear at the final assembled version, these personnel should not be listed on the budget. To add WCM personnel to a proposal without them being listed on the budget, Department should select “consultant-key” in the personnel type and from there enter their specific role – in this case “Sponsor”. For any personnel that is external to WCM, the Department needs to select “external consultant-key”, and then proceed specifying the role.
The PHS assignment form is optional, but it is being requested by the OSRA Specialist. Why do I need to provide this?
While the PHS assignment form is optional, it has two very important functions for review: 1. It allows both Department research administrator and OSRA to confirm that the indented IC participates in the Funding Opportunity and 2. It allows OSRA to ensure that the application follows any IC-specific requirement that may not be included in the RFA (the NCI, for instance, has thresholds for effort commitment that must be met for certain programs). The form is not needed if the RFP is IC-specific.
I am getting an error saying that Attachment Filenames are above 49 characters. Do I need to delete and reupload everything?
By clicking on the Attachment Filenames hyperlink, on the Finalize tab, you can rename any file with a name above 49 characters without having to reupload it! File names above 49 characters are not accepted by the NIH and will generate an error and prevent submission.

A few sections of the application have hyperlink and urls and the PI would prefer not to remove it, but the Specialist advised against. Do they need to be removed?
According to the NIH SF424 (R&R) Application Guide, the use of hypertext (e.g. hyperlinks and URLS) in applications is restricted due to multiple concerns, including reviewer confidentiality, “overstuffing” applications, review consistency, and malware. If the FOA explicitly permits hyperlinks, then you may include them (always read the entire text of the FOA). The following is allowed:
- Hyperlink/url to the senior personnel “MyNCBI” profile is allowed in the NIH biosketch
- When hyperlinks are permitted, in the interest of transparency, the full URL must be presented, as in https://www.nih.gov
- Institutional websites and emails listed on letterheads of letters of support or other letters don’t need to be removed
- There are no special permissions for links to NIH or other government websites
- There is no explicit allowance for links in application sections, such as “Facilities and Other Resources” that do not have page limits
- For biographical sketches, citations that are not covered by the Public Access Policy, but are publicly available in a free, online format may include URLs or PubMed ID (PMID) numbers along with the full reference. Active hyperlinks in this section are not allowed.
Risk avoidance is the most convincing reason to reconsider listing a hyperlink on your proposal, because the NIH may withdraw your application from consideration if they believe the hyperlink is providing additional information. The best approach is to ensure that the application is self-contained.
Do I need to have signed Consortium Statements from subawards?
These are formal arrangement between collaborating sites for a proposal. Commonly referred to as Statement of Intent for Consortium/Consortium Statement, these documents confirm that the information provided for the proposal has been vetted and approved by an institutional officer at the subsite. While NIH does not require the actual SOI to be uploaded, these documents are subject to audit and must be on file at the prime applicant institution prior to submission.
Do all proposals require a cover letter?
Cover letters are not considered part of the application itself and are reviewed by the NIH Center for Science Review only (CSR). In addition to any requirement in the specific FOA, general cover letter requirements are listed here: https://grants.nih.gov/grants/how-to-apply-application-guide/forms-h/general/g.200-sf-424-(r&r)-form.htm#21

