Overview
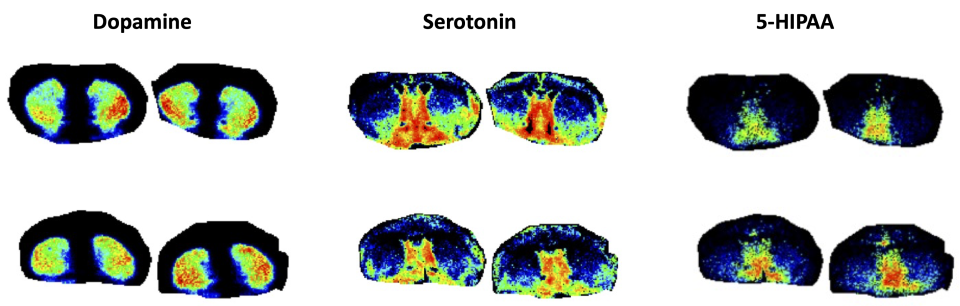
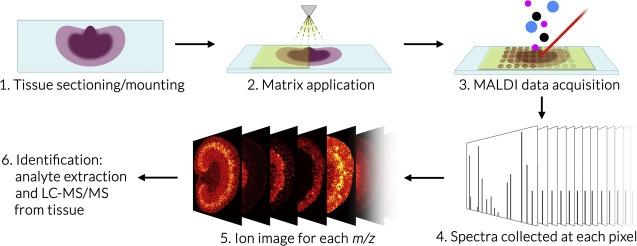
Imaging mass spectrometry (IMS) is a sensitive molecular technique that uses mass spectrometric detection of biomolecules to generate quantitative spatial distribution maps that can be overlaid with optical images. IMS enables the simultaneous analysis of thousands of different molecules such as metabolites, lipids, and peptides in a single experiment without labeling.
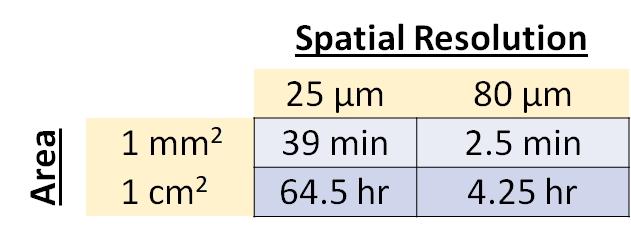
The Bruker SciMax is an ultra-high mass resolution Fourier Transform – Ion Cyclotron Resonance (FT-ICR) mass spectrometer capable of determining fine isotopic structure that enables unambiguous identification of biomolecules. The SciMax uses laser ionization (MALDI) to raster across a tissue slice collecting mass spectra pixel by pixel. Each spectrum contains information that can identify and provide relative quantification for hundreds or even thousands of different molecules. By adjusting the size and spacing of laser pulses, the SciMax can generate images with differing spatial resolutions. We offer high (25 µm) and low (80 µm) resolution.
MALDI Imaging Services are billed by hours of instrument and subsequent analysis time with a modest fee for slide coating with matrix. For high spatial resolution analysis, it is important to identify areas of interest.