What is Just-in-Time?
Just-in-Time (JIT) refers to the application timeframe requiring applicants to send updated information to the NIH only if an award is likely. This process decreases the administrative burden for 75%-80% of the applicants that will not receive funding and provides NIH with the most current information “just-in-time” for award.
Applicants will be notified via email when Just-in-Time information is needed. This notification is not a Notice of Award nor should it be construed to be an indicator of possible funding.
When do I respond?
Applicants will see a JIT link appear on the eRA Commons’ Status screen soon after the review scores are posted but should only submit JIT information when it is specifically requested by the grantor agency. Requests for JIT materials will typically be sent by the Grants Management Specialist to the AOR and PI via email. As of October 1, 2024 NIH will no longer sent automated JIT notifications.
JIT materials should be submitted by the date requested by agency staff.
How do I check my application score?
Only the PI can view the summary statement, score and percentile information for a submitted application. The information is available in the eRA Commons within 30 days of the review of the application.
Below are step by step instructions on how to access review outcomes:
- Log into eRA Commons
- Select the Status tab from the eRA Commons menu
- Click on the List of Applications/Grants section.
- Select the application ID link for the specific application.
What will a JIT request include?
A JIT request will include the following (as applicable):
- Current Other Support for all Key Personnel
- Certification of IRB Approval
- Verification of IACUC Approval
- Human Subjects Education Requirement
- Human Embryonic Stem Cells (hESCs)
- Genomic Data Sharing Institutional Certification
- Other Information:
- Grantor may also request additional JIT information on a case-by-case basis, such as revised budgets or changes to the human subjects or vertebrate animal sections of the application.
What is the submission process?
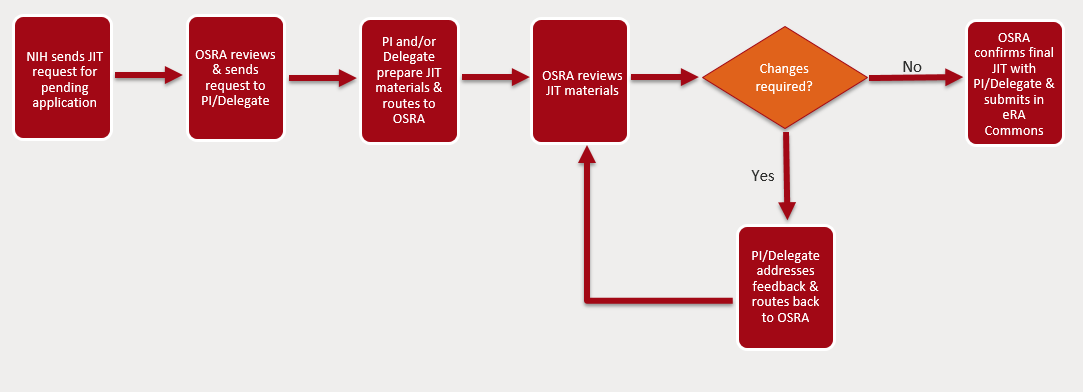
NIH JIT requests are submitted via the JIT link in the Status screen of the eRA Commons. PIs can upload and save JIT information, but only OSRA has the authority to submit to the NIH.
Below are the steps for the submission process:
- NIH sends a JIT request to OSRA via email.
- OSRA reviews and sends JIT Request notification via email to the PI and their delegate(s).
- The PI and/or their delegate prepares all applicable JIT materials and routes to OSRA via e-mail. Materials should be routed to OSRA within 4 business days of the JIT deadline to ensure sufficient time to review. JITs for Multi-component applications and applications with many key personnel should be routed to OSRA 7 business days before the deadline given the volume of information to review.
- OSRA reviews JIT materials
- If changes are required, OSRA will provide feedback to PI and their delegate via e-mail. The PI and/or delegate can then route the JIT materials back to OSRA for review.
- If no changes are required, OSRA will confirm approval to submit final JIT materials to NIH with the PI and/or delegate.
- OSRA uploads final JIT materials to eRA commons. Once submitted OSRA will provide submission confirmation to the PI and their delegate.
Click here to review OSRA’s full Standard Operating Procedure on Sponsor Pre-Funding "Just in Time" Requests.
When should I start my protocol submission to the IRB and/or IACUC?
If your application scores within or near the NIH Institute or Center published paylines or near the previous fiscal year published paylines, as soon as you receive the first auto-generated JIT email request you should begin the protocol/submisison amendment process.
Special Considerations
- IRB Approval – WCM IRB approval must be acquired for human subjects research funded through WCM regardless of where the research is performed. The PI/Department must submit a new protocol application or add the funding source to an existing protocol as soon as they know a proposal is likely to be funded to ensure the IRB office is able to review and approve the proposed work prior to JIT submission. If WCM is prime and all human subjects work is occurring at a subsite, acknowledgement from WCM’s IRB must still be obtained. If using a NonWCM/external IRB, an acknowledgement letter from WCM IRB is needed before the study may be initiated at WCM. Once approved by the IRB of Record a submission must be made within 2 weeks for WCM acknowledgement. Please review IRB’s Single IRB Reliance website for additional guidance on this process.
- IACUC Approval – WCM IACUC approval must be acquired for animal research funded through WCM regardless of where the research is performed. The PI/Department must submit a new protocol or protocol amendment as soon as they know a proposal is likely to be funded to ensure the IACUC office is able to review and approve the proposed work prior to JIT submission. If the animal work proposed will all occur at a collaborating subsite, the PI/Department must complete the IACUC Collaboration Form and submit to the IACUC office. WCM IACUC concurrence is required on all proposed animal subjects work at a collaborating site.
- Genomic Data Sharing (GDS) Certifications –Investigators working with large-scale human genomic data are required to submit a GDS Institutional Certification to NIH before an award can be issued. If WCM’s IRB Office has already approved the proposed study, then the Institutional Certification form must be completed and routed to the IRB office for concurrence. Once approved, the form can be routed to OSRA for AOR signature and submission. Confirmation of IRB concurrence must be provided along with the form. If the proposed study is pending IRB approval, then the Provisional Certification form must be completed and routed to OSRA for AOR signature.
Helpful Links

