Generally, grants, contracts, cooperative agreements, and other funding mechanisms require regular reporting of the progress of the project through periodic financial and technical reports. The format, frequency, and depth and breadth of the reporting are determined by the sponsors. Principal Investigators (PIs) and their Departmental Staff are responsible for thoroughly reviewing all award notices for full progress report requirements and details.
NIH Research Performance Progress Report (RPPR)
The Research Performance Progress Reports (RPPR) is the progress report form used by grantees to submit progress reports to the NIH. The RPPR documents grantee/recipient accomplishments and compliance with the terms of the award. There are three types of RPPRs, all of which use the NIH RPPR Instruction Guide.
Annual RPPR
The Annual RPPR is used to describe to NIH a grant’s scientific progress, identify significant changes, report on personnel, and describe plans for the subsequent budget period or year (if applicable). Submission of the RPPR is required in order for the next year of funding to be released by NIH.
Due Dates
- Streamlined Non-Competing Award Process (SNAP) RPPRs are due approximately 45 days before the next budget period start date.
- Non-SNAP RPPRs are due approximately 60 days before the next budget period start date.
- Multi-year funded (MYF) RPPRs are due annually on or before the anniversary of the budget/project period start date of the award
OSRA sends RPPR e-mail reminders to Principal Investigators and their administrative support team 60 days in advance of the due date.
Interim RPPR
The Interim RPPR is required when submitting a renewal (Type 2) application. If the Type 2 is not funded, the Interim RPPR will serve as the Final RPPR for the project. If the Type 2 is funded, the Interim RPPR will serve as the annual RPPR for the final year of the previous competitive segment. The data elements collected on the Interim RPPR are the same as for the Final RPPR, including project outcomes.
Due Date
One hundred and twenty (120) days from period of performance end date for the competitive segment.
Final RPPR
The Final RPPR is part of the award closeout process and provides information on the project outcomes in addition to the information submitted on the annual RPPR, except budget and plans for the upcoming year.
Due Date
One hundred and twenty (120) days from period of performance end date for the competitive segment.
Internal Routing and OSRA Review Process
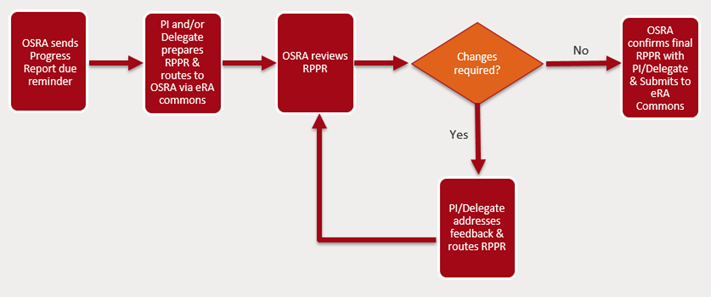
All RPPRs are submitted via the eRA Commons.
- Upon receipt of the e-mail reminder from OSRA, the RPPR should be initiated by the PI in the Commons to allow for Department delegates to start collecting the required documents and information.
- Once all documents and information have been finalized, the RPPR should be routed to the signing official for WCM, Aleta Gunsul. Departments must ensure RPPRs are free of errors prior to routing. If assistance is needed, the assigned Specialist should be contacted.
- The OSRA assigned Grants Specialist will review and:
- If revisions are required, OSRA will provide feedback to the Department and return the RPPR via Commons for edits;
- If revisions are not required, OSRA will submit the RPPR and share a copy with PI and Department, upload a copy on the WRG record, and update the status to “Progress Report Submitted”
RPPRs must be routed to OSRA for review 7 business days before the deadline. Multi-Component RPPRs are due 10 business days before the deadline. OSRA Specialists will do their best to prioritize review and submission of reports routed after the internal deadline, however that will depend upon volume. RPPRs will be overdue until reviewed and submitted. Requests for submission without OSRA review will not be accommodated.
Click here to review OSRA’s full Standard Operating Procedure on Submitting a Progress Report for OSRA's Review.
Helpful Links
DOD and NSF Progress Reports
Monthly/Quarterly/Semi-annual or reports other than annual and final are based on the terms and conditions outlined in the award agreement. Departments and PIs need to refer to the award agreement for specific content and formatting requirements.
These reports are technical in nature and do not require AOR review and approval. Principal Investigators and Departments are responsible to prepare and submit the reports directly via www.ebrap.org (for DOD awards) or www.research.gov (for NSF awards) by the timeline outlined in the Notice of Award.
Non-Federal/Foundation Progress Reports
Progress report requirements, including content, submission method and deadline frequency, can vary from sponsor to sponsor. Principal Investigators (PIs) and their Departmental Staff are responsible for thoroughly reviewing all award notices for full progress report requirements and details. Progress reports that require AOR submission and/or signature must be routed to the OSRA Specialist for review and submission 7 business days before the deadline. The Specialist will review and provide comments or proceed with submission/signature and return a confirmation to PI and Department.
Progress reports that do not require AOR submission are to be prepared by the PI with Department staff support in accordance with the sponsor requirements and should be submitted directly to the sponsor according to the guidance and timeline established in the award agreement. A copy of the submitted progress report should be forwarded to the assigned OSRA Specialist so that a copy can be uploaded into the WRG PT for record retention.

