Endothelial cells with a distinct gene-expression signature play a key role in the development and repair of blood vessels throughout life, according to a new study by Weill Cornell Medicine investigators.

Dr. Yang Lin
The study, published April 29 in Circulation, helps solve a long-standing mystery in cardiovascular research. Scientists suspected that the blood vessels, which are lined with cells called endothelial cells, harbor cells that help with regeneration and repair as many other organs and tissues do. Now, the first author Dr. Yang Lin, an instructor of regenerative medicine and a member of the Hartman Institute for Therapeutic Organ Regeneration at Weill Cornell Medicine, and colleagues show that a subset of endothelial cells acts as a reservoir for repair and regrowth during development and after injury. They show these cells can be distinguished from other mature endothelial cells by their high expression of a gene called ATP binding cassette subfamily G member 2 (ABCG2).
Dr. Shahin Rafii, chief of the Division of Regenerative Medicine and the Arthur B. Belfer Professor in Genetic Medicine at Weill Cornell Medicine, and Dr. Mervin Yoder, Distinguished Professor Emeritus at Indiana University School of Medicine, co-led the study with Dr. Lin.
“Specific subsets of ABCG2-positive endothelial cells proliferate when a blood vessel needs to grow or be repaired,” Dr. Lin said. “Learning more about these unique ABCG2-expressing cells could lead to new therapies for cardiovascular diseases.”

Dr. Shahin Rafii
Endothelial cells line the insides of blood vessels and help transport nutrients and oxygen around the body. When they become aged or injured, they may not function well, contributing to conditions like cardiovascular disease or metabolic disease, Dr. Lin said. Most scientists thought these cells were all the same and together regenerate new cells for growth and repair, he said.
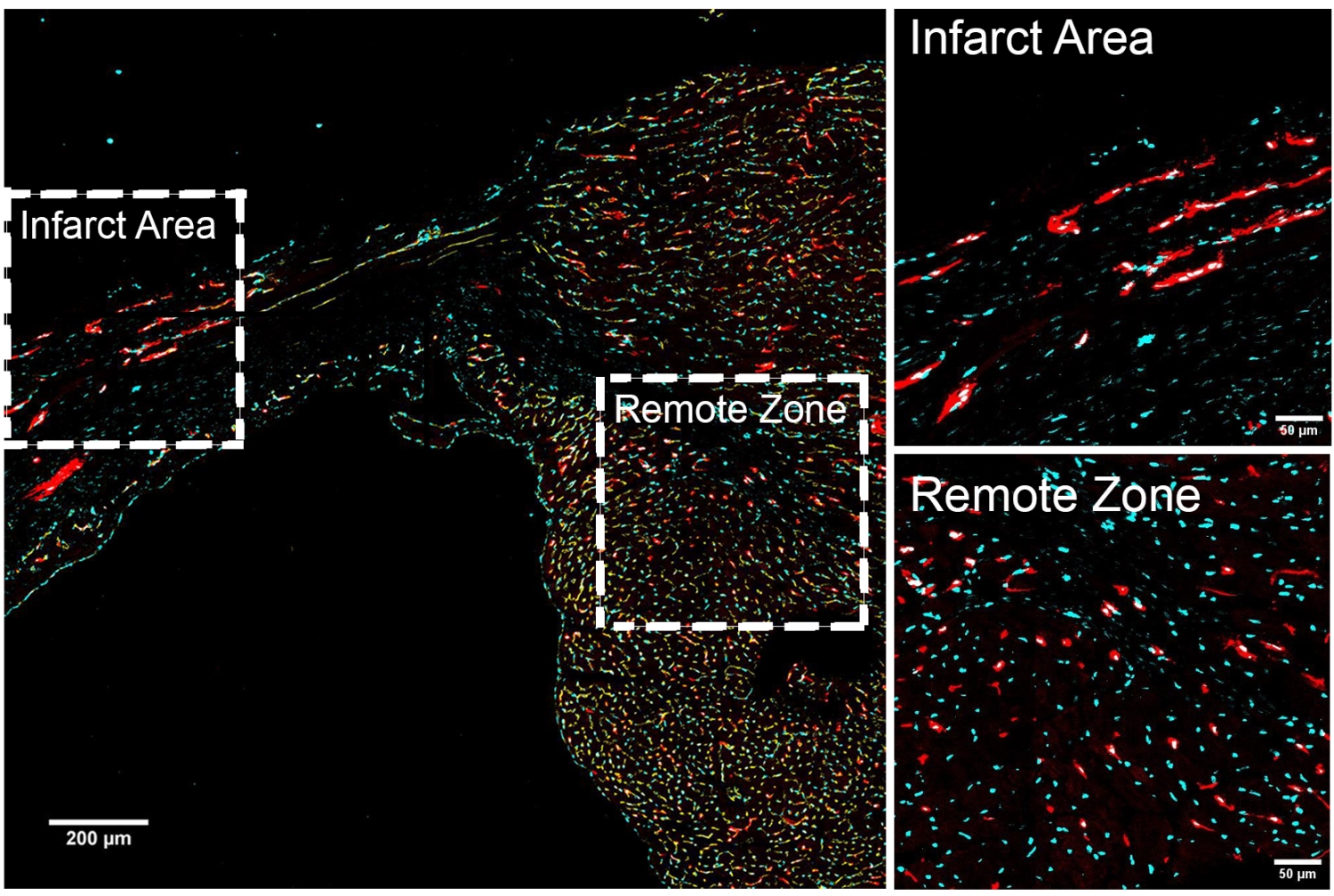
However, Dr. Lin and his colleagues found that one subgroup of endothelial cells has more regenerative capacity than others. The team showed that ABCG2-expressing endothelial cells drive the formation of veins, arteries and capillaries in development. They also showed in animal experiments that these ABCG2-expressing cells spring into action after a heart attack to repair damaged blood vessels.
The investigators next determined how these regenerative cells are genetically regulated. They identified the ABCG2-expressing cells’ genetic signature by analyzing which genes are turned on and off in the cells and how gene activity is controlled. The analysis also gave the investigators insights on the genes that contribute their healing abilities. For example, they found genes very active in regenerative cells throughout the body are also active in the ABCG2-expressing cells, as are genes important in blood vessel development.
“In the future, we would like to learn more about how these cells maintain their potential for self-renewal throughout life,” he said.
Indeed, their discoveries could lead to the development of therapies for diseases that affect the heart and blood vessels. Dr. Lin explained that engineered ABCG2-expressing cells could be grown in the laboratory and transplanted into patients to help heal damaged blood vessels. Scientists could also develop drugs targeting these cells.
“We could create cellular therapies with these ABCG2-expressing cells or drugs targeting them to treat cardiovascular disease or promote blood vessel regeneration,” Dr. Lin said.
Dr. Lin said he hopes the study will also help other scientists appreciate the diversity of endothelial cells. “Endothelial cells are not all the same,” Dr. Lin said. “There are different endothelial cell types, and we need to precisely target select ones when we develop new therapies.”
Many Weill Cornell Medicine physicians and scientists maintain relationships and collaborate with external organizations to foster scientific innovation and provide expert guidance. The institution makes these disclosures public to ensure transparency. For this information, see profile for Dr. Rafii.
The research reported in this story was supported in part by grants from the National Heart, Lung and Blood Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, and the National Institute of Allergy and Infectious Diseases, all part of the National Institutes of Health, through grant numbers R35HL150809, R01HL171277, RC2DK114777 and U01AI138329. Additional funding was provided by the Empire State Stem Cell Board (NYSTEM; C030160); the Hartman Institute for Therapeutic Organ Regeneration, the Ansary Stem Cell Institute. the Daedalus Fund for Innovation, Selma and Lawrence Ruben Science to Industry Bridge Fund at Weill Cornell Medicine; and the Starr Foundation (TRI-SCI 2019-029).
