
Two highly similar molecules with essential, but often contrasting, signaling roles in most life forms exert their distinct effects through subtle differences in their bindings to their signaling partners, according to a new study led by researchers at Weill Cornell Medicine.
In the study, published March 27 in Nature Structural & Molecular Biology, the researchers used exquisitely sensitive measurement techniques to reveal at the single-molecule level how the signaling molecules cAMP and cGMP bind to an ion channel from the pacemaker channel family, one of the major types of proteins whose activities they regulate.
Ion channels are common features of cell membranes, and control basic cell functions by allowing calcium, sodium, potassium and other charged elements, called ions to flow in and out of cells. Many ion channels can bind both cAMP and cGMP while being effectively switched open by only one of them. Precisely how the two molecules exert their differing effects on ion channel activity had been a mystery. The study details how cAMP/cGMP bind to ion channels and advances the understanding of a fundamental aspect of cell biology. These findings could eventually inspire new treatments for disorders involving ion channel malfunctions.
“We found clear differences in the interaction and binding strength of these two molecules to the ion channels, which we think explains why one can open the channel and the other cannot,” said study senior author Dr. Simon Scheuring, a professor of physiology and biophysics in anesthesiology at Weill Cornell Medicine.
Cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) are known as cyclic nucleotides: “cyclic” because their chemical structures contain cyclic or ring motifs, and “nucleotides” because they are part of the same family of molecules as the nucleotide building blocks A and G of DNA. They appear to have evolved as multipurpose switches capable of regulating the activity of a wide range of different protein targets. Often only one of them, cAMP or cGMP, is the activator, while the other does little or nothing directly to the target but can force it into an inactive state by binding to the same site, thus the competition between the two molecules switches the channels on and off.
The proteins regulated by cAMP/cGMP include a large class of ion channels called cyclic nucleotide-gated (CNG) ion channels. CNG channels have important roles across the nervous system, including in the sensory neurons mediating smell and vision, and in the pacemaker cells that govern heartbeat rhythm. Dr. Scheuring, who has helped pioneer the use of a sensitive measurement technique called atomic force microscopy (AFM), and Dr. Crina Nimigean, an ion channel expert and a professor of physiology and biophysics in anesthesiology at Weill Cornell Medicine, have already made considerable progress in understanding how cAMP/cGMP regulate CNG channels. In a 2018 paper, for example, they used high speed AFM to show how a bacterial CNG channel, SthK changes conformation when bound by the channel-opener cAMP or the effective channel-closer cGMP.
In the new study they teamed up again and were also joined by a molecular-dynamics modeling expert Dr. Helmut Grubmüller of the Max Planck Institute for Multidisciplinary Sciences in Germany. Their main technique this time was an AFM-related force-sensing method called AFM single molecule force spectroscopy, which is sensitive enough to measure the binding force of just one cAMP or cGMP molecule to its binding site on the ion channel. With that, and help from computational modeling, they quantified how cAMP and cGMP differ in their binding strengths and depth to the same binding site on SthK, via interactions with different clusters of atoms within the binding site.
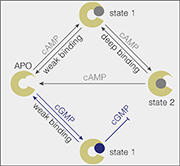
Model of how cAMP and cGMP bind to the SthK cyclic-nucleotide binding domain.
“Cyclic AMP can access a more strongly bound state; that is, it stays longer in its binding site on the ion channel, compared with cGMP, suggesting that this deep-bound state is the key to channel activation,” said study first author Dr. Yangang Pan, a postdoctoral researcher in the Scheuring laboratory.
The SthK channel is just one model for mammalian CNG channels, and the researchers plan future studies with mammalian CNG channels. But they believe that their SthK findings already illuminate the fundamental mechanism of how cAMP and cGMP work as regulators in their many roles throughout biology.
“Binding sites for cAMP/cGMP are found not just on ion channels but also on signaling enzymes, transcription factors, and other proteins,” Dr. Nimigean said. “We suspect that in every case, nature has tuned how these proteins recognize cAMP/cGMP, in accordance with these proteins’ functions.”
