Core Laboratories Center (CLC)
Core Facilities
- CLC Genomics and Epigenomics Core Facility
- CLC Proteomics and Metabolomics Core Facility
- CLC Flow Cytometry Core Facility
- CLC Microscopy and Imaging Core Facility
- CLC Citigroup Biomedical Imaging Center
- CLC Nuclear Magnetic Resonance Core Facility
- CLC Milstein Chemistry Core Facility
Summary
Current and emerging biomedical technologies present exceptional opportunities for novel and creative research with the potential for breakthrough discoveries. To support such research efforts, the Weill Cornell Medical College (WCMC) Core Laboratories Center (CLC) offers a coordinated array of cutting-edge biomedical technologies, expertise in their applications, technology testing and development, educational workshops, and training and consultation to the university's research community and to outside investigators. The Center currently has core facilities that provide resources and services for genomics and epigenomics, proteomics and metabolomics, flow cytometry, imaging (e.g., confocal microscopy, multiphoton, electron microscopy, optical CT, ultrasound, MRI, and PET), nuclear magnetic resonance, and synthetic chemistry. Additional core facilities are expected soon. The mission of the CLC is to promote biomedical research with advanced technologies in a shared resource environment. Implementation of high end biotechnologies with multi-disciplinary support in a core facility center enables cost effective access and broad based use of these technologies, with high impact results. The resources and services of the CLC are open to all investigators at Weill Cornell Medicine, Cornell University and Cornell-affiliated institutions. The Center also provides services to external investigators at both academic institutions and commercial enterprises. The CLC supports many local, national and international collaborations. With a concentration of advanced biotechnologies and a broad investigator user base, the CLC is a dynamic hub for biomedical research.
More Information
For more information about all CLC services and pricing, to register as a user of any CLC core facility, to submit samples and/or to schedule use of instruments, please go to the WCM iLab portal.
Emergency Freezers:
The Medical College has available two -80°C Revco freezers for emergency short-term (i.e., approx. 1 week) use by investigators who experience a freezer breakdown that requires repair. This service is intended to help minimize the loss of valuable reagents and samples in the event of a -80°C freezer breakdown. Emergency protocols established by the Maintenance and Engineering Office should be followed in the event of a freezer emergency.
High Throughput Screening Resource Center
The High-Throughput Screening Resource Center (HTSRC) supports scientists in the screening and identification of compounds and genetic modulators of in vitro assays which utilize optical and other bio-analytical technologies. High-Throughput Screening (HTS) requires the miniaturization and automation of bioassays so that millions of variables can be tested. HTS is often an important step in the discovery of new medicines. The center has a collection of 169,000 compounds, automated liquid transfer devices, compound databases, and supports a broad diversity of assay development techniques, typically found in early drug discovery programs.
Visit the High Throughput Screening Resource Center.
Biostatistics and Epidemiology Consulting Service
This consulting service is comprised of a broad collection of services used to identify research questions, construct hypotheses, design investigative studies and data collection strategies, conduct statistical analyses of data, and interpret findings. The Biostatistics and Epidemiology Consulting Service has a proven track record in attracting funding for Weill Cornell laboratories. The inclusion of the consulting service in a number of successful grant applications provides needed expertise to implement and conduct research projects in a cost effective manner. The existence of the consulting service not only strengthens grant proposals, increasing the likelihood of being funded, but also offers our investigators an alternative to using outside consultants.
The Biostatistics and Epidemiology Consulting Service is run by the Division of Biostatistics and Epidemiology of the Department of Healthcare Policy and Research. [Please see the Division website for more information.]
The Research Data Storage Core Facility
What is it?
The Research Data Storage Core (RDSC) Facility provides secure networked storage space for electronic research data for investigators. This service will be free for up to 25 GB. For amounts over 25 GB fees will apply. An additional 3 TB limit per Principal Investigator will apply.
This service will be free for up to 25GB. For amounts over 25 GB additional fees will apply.
What projects qualify?
As the name implies, only research data may be stored here. The RDSC is especially useful for large research data files, such as research imaging, microarray and sequencing data.
For other documents such as administrative, clinical, or financial information investigators are urged to use their department's server. Please contact your department administrator for more information on department servers.
Is the data backed up?
No.
Who will be allowed access to an investigator's folder?
The research investigator will decide who in their research team can access their folder. Since this is intended only for research data, there will be no hierarchical access to the data unless specifically required for that project. In other words, everyone in the designated research group will be able to access all the information within the research folder. Moreover, other researchers who are users of the RDSC will be able to drop large files into a designated "Dropbox" folder (write-only access) allowing for collaboration between different research groups.
How do I get started?
Please download the forms for new requests to get started using this service.
RDSC New Request (pdf)
RDSC Change Request (pdf)
Once the requests are approved you will be contacted to arrange for setup.
Do I need more bandwidth to use this service?
This service does not require any special wiring. The bandwidth (or the speed) of your connection depends on the wiring in your location which is in the range of 10 Mbps to 1 Gbps.
What is the Dropbox folder?
Once your storage space is setup, you will log in and notice one of the main folders is named "dropbox". This folder allows data sharing between different groups that are members of the RDSC. Individuals in your group have full access to this folder, while individuals from other groups in the RDSC have write-only access to this folder (i.e. they cannot see the contents of the folder after dropping a file into it).
Can I access this service remotely?
Yes. This service is accessible via the Cisco Virtual Private Network (VPN). The Weill Cornell Web VPN cannot be used to access this service. For more information or assistance on remote connectivity please visit the Remote Access section of the Weill Cornell ITS web site, or e-mail the Help Desk at support@med.cornell.edu.
Support
ITS will only support connectivity to the file server using the CIFS protocol (Common Internet File System) from ITS certified operating systems (Windows XP, Windows 7 and Mac OS 10.5 and later). Any issues that are not new requests or change requests should be directed to support@med.cornell.edu.
Gene Therapy
The Belfer Gene Therapy Core Facility provides the infrastructure to carry out basic, translational and clinical research utilizing gene transfer.
Facilities
The Belfer Gene Therapy Core Facility is a new, fully equipped core facility devoted exclusively to developing and assessing gene transfer vectors. The Vector Core includes 6 independent cell culture rooms plus bench and desk space for 8 scientists, an office for the Director and an instrument room containing large equipment. The Director supervises a technical staff of 9 (7 technicians and 2 postdoctoral associates) individuals. The Vector Core includes analytical resources: quantitative PCR, 2 HPLC's, plate readers, automated liquid handling devices, phosphor-/fluoro-imager and appropriate computer support and backup. There is also a database of the available vectors and plasmids with extensive sequence and restriction mapping data. The Good Manufacturing Practice (GMP) Core is comprised of the GMP facility which occupies approximately 2,400 square feet devoted exclusively to production of gene transfer vectors and gene modified cells for human therapeutic trials. There is office space and supporting laboratory space for analyses and quality control of GMP grade vectors including a room with a Biostat C fermentor. The design, construction, and validation of the GMP production facility is monitored and reviewed with the FDA to assure full compliance with all current GMPs. The GMP facility consists of a suite of three fully equipped production rooms, a staging area, gowning and degowning pathways laid out with a series of interlocking double-door pass-through devices and doorways. In conjunction with carefully maintained differential room pressure relationships and single pass HEPA filter air, standard operating procedures define the flow of material, personnel, and waste through the facility and particulates and equipment are monitored full time. In addition, there are separate rooms for QC released and quarantined materials, a cell bank and a quality control lab. There is an extensive set of standard operating procedures for the use, cleaning, and validation of equipment, and for the purchase, tracking, and storage of supplies and for training personnel.
Services
The Vector Core functions as a resource to investigators to provide centralized expert services and training in the design, creation, and production of gene transfer vectors, and to provide a characterized repository of gene transfer vectors and related reagents for use by investigators. The primary resource of the Vector Core is the expertise to efficiently design, construct and produce gene transfer vectors, including adenovirus (Ad), adenoassociated virus (AAV), lentivirus, retrovirus vectors, as well as non-viral vectors and plasmid vectors. New cDNA's of biological significance are continuously being acquired and used to make first generation and more advanced gene transfer vectors. The Vector Core maintains, and continually updates, standard operating procedures for the construction, production, and verification of gene transfer vectors. Many individual steps of vector production have been optimized and new protocols and reagents are evaluated and incorporated into the standard operating procedures.
Genetically Modified Animal Phenotyping

The decoding of the human genome and advancements in scientists' abilities to manipulate the mouse genome have resulted in the generation of countless mouse models of human disease and tools to dissect the function of specific genes. It is expected that the numbers of genetically engineered mice carrying transgenes, targeted mutations, and chemically-induced mutations will only continue to increase.
The Genetically Modified Animal (GMA) Phenotyping Service exists to serve investigators at Memorial Sloan-Kettering Cancer Center, the Rockefeller University, and the Weill Medical College of Cornell University by providing an extensive baseline phenotypic profile of genetically engineered animals. Such a comprehensive baseline characterization will be invaluable to investigators unfamiliar with normal anatomy, histology, physiology and age- or strain-related background lesions of their research species. Evaluating the entire animal, as opposed to a specific tissue or organ system, will help to identify unanticipated phenotypic changes.
Services
Genetically Modified Animal Phenotyping
This includes one or more of the following:
- Hematology
- Complete Blood Count
- White Blood Cell Differential
- Clinical Chemistry
- 27 Biochemical Assay
- Urinalysis
- Specific Gravity
- Colorimetric Test for 10
- Parameters
- Sediment Analysis
- Survey Radiographs
- Lateral and Dorsal-Ventral Views
- Gross Necropsy
- Whole Mouse and Parenchymal Organ Weights
- Bone Marrow Smear Evaluation
- Extensive Microscopic Evaluation
- Digital Images of Macroscopic and Microscopic Lesions
- Electronic Report
- Interpretive Summary
- Recommendations for Ancillary Analyses
Other Services
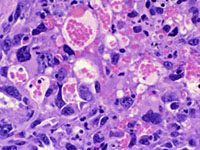
Subcutaneous hemangiosarcomas in a P53 Homozygous knockout mouse. Hematoxylin and eosin, 40X. Courtesy of Krista La Perle, DVM, PhD
Complete histology services including tissue processing, paraffin embedding, cryostat services and staining with routine stains such as hematoxylin & eosin and special histochemical stains are available. Immunohistochemistry, immunofluorescence, and digital slide scanning are available also through the LCP. The GMA Phenotyping Service has an MX-20 Faxitron , which produces extremely high-resolution radiographs of small laboratory animals (mice and rats), as well as excised tissues and paraffin blocks. Specimens can be placed on adjustable shelves to obtain images magnified up to 5x.
Submission Process
Click here to download a PDF of the presubmission form. The form should be filled out completely.. The resulting phenotypic profile will be greatly enhanced by the extent of the experimental details provided, including tissue- or age-specific gene expression and observed/expected phenotypes. The information provided will enable the GMA Phenotyping Service staff to recommend the age, gender and number of mice required to adequately characterize your mice. However, it is typically recommended that at least two male and two female mice of each genotype, approximately 8-12 weeks of age, be submitted alive. Submitters will need to meet with a LCP pathologist prior to any phenotyping projects. Email LCP@mskcc.org to schedule an appointment. Please do not submit animals unless prior arrangements have been made, as the laboratory cannot house live animals.
Mouse Genetics Core Facility
WCMC provides investigators access to the services offered by the MSKCC Mouse Genetics Core Facility.
The primary role of the Mouse Genetics Core Facility (MGCF) is to facilitate the use of mouse molecular genetics for in vivo studies of gene functions in biomedical research. Biological problems investigated using the services and expertise of the MGCF include cell survival and proliferation, embryonic development and cell differentiation, organogenesis, cell-cell interactions, immune responses, and the generation of mouse models of cancer.
The MGCF consists of three interacting groups: the Transgenic/Embryology Group (TXG), the Tissue Culture Group (TCG), and the Colony Management Group (CMG). The MGCF performs work both at the Long Island City facility and on the main campus in Manhattan.
Services
The Transgenic/Embryology Mouse Group
- Purification of transgene DNA
- Production of transgenic mice
- Genotyping of transgenic founders
- Injection of ES cells into blastocysts
- Chimera Breeding
- Cryopreservation
- Rederivation
- Special procedures
- Provision of special strains
- Gene Editing by TALENs & CRISPRs
The Tissue Culture Group
- Assist users in all aspects of ES cell culture, gene targeting, and ES cell establishment
- Provision of mouse embryonic fibroblasts (MEF) and ES cell stocks
The Colony Management Group
- General husbandry service
- Comprehensive Colony Management
Service Requests
Please use iLab to request services.
Fees
Download the 2014 Fee Schedule.
The Starr Foundation Tri-Institutional Stem Cell Derivation Laboratory
The Starr Foundation Tri-Institutional Stem Cell Derivation Laboratory (SCDL) at Weill Cornell Medical College is a shared lab and support facility that serves researchers working with pluripotent stem cells. Our main goal is to provide centralized access to multiple technologies that enable the derivation, differentiation, characterization, isolation and functional assessment of pluripotent stem cells and their tissue-specific derivatives.
The SCDL provides a range of functions that are focused in these key areas:
Pluripotent Stem Cell Banking
TThe SCDL serves as a repository for more than 40 human Embryonic Stem Cell (hESC) lines that were derived from blastocysts donated following fertility treatment at Weill Cornell Medical College. Many of these lines were developed from embryos carrying genetic mutations identified by preimplantation genetic diagnosis (PGD), thus providing pluripotent stem cell lines that may be used to model genetic disease. Furthermore, new technological approaches to somatic cell nuclear transfer (SCNT) to generate pluripotent stem cell lines from skin biopsy have been investigated for potential future derivation of patient-specific stem cells that could be applied in regenerative therapies. Click here for the list of WMC lines. [^attachment-name.zip]. Nikica Zaninovic, Ph.D. and Qiansheng Zhan, Ph.D. as WMC hESC investigators.


Pluripotent stem cell banking
A 7 day old human embryo (blastocyst) is shown at the left with nuclei pseudo-colored to identify unique cellular sub-types. At this stage of development, the first distinctions between unique cell types is made, with trophoblast shown in red, primitive endoderm shown in green and the inner cell mass shown in blue. Inner cell mass ultimately generates all cell types in the embryo and can be isolated and cultured in vitro to generate embryonic stem cells (shown at right). These embryonic stem cells can be propagated indefinitely in vitro while retaining the ability to differentiate to all the specialized cells/tissues of the body in vitro.
Live Imaging
An imaging suite adjacent to the tissue culture facility contains three Zeiss inverted microscopes capable of live image capture; an Apotome system using a LED based Colibri light source; a 510META Confocal Laser Scanning Microscope (LSM) with five laser lines; and a 710META Confocal LSM with six laser lines. All three microscopes are equipped with stage mounted incubation devices that enable precise environmental control (CO2, O2, humidity, temperature) during live image capture.
Live Imaging Core Facility Access times and Fee
510 META
Monday - Friday 9am-5pm
$40/hour for extramural users with discounts for active Starr Foundation Tri-SCI funded users.
Off hours (by appointment only)
$80/hour for extramural users with discounts for active Starr Foundation Tri-SCI funded users.
710META
Monday - Friday 9am-5pm
$75/hour for extramural users with discounts for active Starr Foundation Tri-SCI funded users.
Off hours (by appointment only)
$125/hour for extramural users with discounts for active Starr Foundation Tri-SCI funded users.
Training $200 per individual.



Live imaging
On the left, a transgenic mouse embryo at day 8 post-fertilization is shown. This unique transgenic mouse strain enable specific labeling of endothelial cells (green) and cells that arise from heart progenitors (red). Nuclei (grey) and a marker of the primitive heart field (blue) are also shown. On the right, a similar transgenic embryo is shown at day 10.5 post-fertilization with endothelial cells shown in green and heart cells shown in red.
Flow Cytometry and Sorting
A BD LSRII Flow Cytometer and a FACS ARIAII Cell Sorter are also available within the SCDL. Each device is capable of measuring up to 22 parameters on cells at a flow rate of up to 20,000 cells per second. The ARIAII can sort for distinct populations and captures cells in sterile conditions that are suitable for transfer to sustained in vitro tissue culture.
Flow Cytometry Core Facility Access Times and Fees
BD LSRII FLOW CYTOMETER
Monday - Friday 9am-5pm
Unassisted: $40/hour for extramural users with discounts for active Starr Foundation Tri-SCI funded users.
Assisted: $75/hour for extramural users with discounts for active Starr Foundation Tri-SCI funded users.
Off hours (by appointment only)
Unassisted: $60/hour for extramural users with discounts for active Starr Foundation Tri-SCI funded users.
Assisted: $100/hour for extramural users with discounts for active Starr Foundation Tri-SCI funded users Training $150 per individual
Cell Sorting Core Facility Access Times and Fees
FACS (BD FACSARIAII)
Monday - Friday 9am-5pm
$80/hour for extramural users with discounts for active Starr Foundation Tri-SCI funded users.
Off hours (by appointment only)
$120/hour for extramural users with discounts for active Starr Foundation Tri-SCI funded users.

Flow cytometry and sorting
A single heterogeneous population of cells was labeled using antibodies that were conjugated to multiple different fluorescent molecules. Using the FACSAriaII or LSRII instruments that are capable of distinguishing up to 22 parameters on a cell, multiple phenotypically unique populations can be distinguished among blood cells derived from human embryonic stem cells. In this example, megakaryocyte (green), erythroid (yellow) and myeloid (pink and red) cells are shown, along with multipotent progenitor cells (blue) that generate all of the above populations.
Director: Zev Rosenwaks, M.D., Co-Director: Shahin Rafii, M.D.
Lab Management: Daylon James, Ph.D., Nikica Zaninovic, Ph.D.
For more information or to schedule services, contact the lab at (212) 746-5985 or SCDL@med.cornell.edu
The SCDL of Weill Cornell Medical College is located at 1300 York Ave, A-803 NY, New York 10065
Iodination
In order to comply with environmental regulations by minimizing all radioactive discharges, an activated charcoal hood designed to trap radioactive iodine was installed for use by investigators. The room is also equipped with a state-of-the-art gamma counter for samples counting at the convenience of the investigator.
Facilities
A dedicated, secure hood rated for iodination purposes for use by licensed investigators at no charge.
Services Provided
Iodination hood and gamma counter.
CLC Advanced Biomedical Technology Seminar Series
Summary
The goals of the Weill Cornell Medicine (WCM) Core Laboratories Center (CLC) Advanced Biomedical Technology Seminar series are (1) to provide the WCM and nearby research community with information about currently available core facility resources and services; (2) to introduce new and emerging technologies and applications, and (3) to provide a public forum to help evaluate if there is a critical mass of interest and potential use for proposed new platforms and possible new services in the CLC core facilities. These seminars are open to all.
List of Previous Seminars
2015
- How Nucleic Acid Mass Spectrometry Complements the Needs of Modern Translational Research. Charles Cantor, Ph.D., Agena Bioscience, 10/30/15.
- A Revolution in Molecular Profiling: NGS-based Gene Expression Using the HTG EdgeSeq Library Prep System. B.J. Kerns, HTG Molecular, Rockefeller University, 11/12/15.
- Unlock Powerful Genomics Insights with Linked-Reads: The 10X Genomics GemCode Platform. Neal Shea, 10X Genomics, 12/8/15.
- Rapid Analysis and Interpretation of Next Generation Sequencing Data: CLC Biomedical Genomics Workbench & Ingenuity Variant Analysis. Robert Mervis, Qiagen Advanced Genomics, 12/16/15.
2016
- CRISPR & RNAi & Small Molecule Screening: Functional Genomics Tools for Translating Biological Entities into Druggable Targets. Ralph Garippa, Ph.D., Memorial Sloan Kettering Cancer Center, 1/7/16.
- Comprehensive Views of Genetic Diversity with Single Molecule, Real-Time (SMRT) Sequencing. Alix Cruse, Pacific Biosciences, 1/19/16.
- High-Throughput Screening and Spectroscopy Resource Center. J. Fraser Glickman, Rockefeller University, 2/5/16.
- Fundamentals and Advancements in Liquid Chromatography–Mass Spectrometry (LC/MS). John Van Antwerp, Waters Inc., 2/9/16.
- Quantitative Metabolic Profiling: On the Critical Path for Understanding Cancer Metabolism. Takushi Oga, Ph.D., Human Metabolome Technologies (HMT), 2/11/16.
- Revolutionize Your Confocal Imaging Session. Overview of the Imaging Facility, Lee Cohen-Gould & Sushmita Mukherjee, Ph.D., Weill Cornell Medicine. Imaging with the LSM 880 and Airyscan, Samantha Fore, Ph.D., Carl Zeiss Microscopy. 3D Imaging of Membrane Traffic, Frederick Maxfield, Ph.D., Department of Biochemistry, Weill Cornell Medicine. 2/12/16.
- Biomarker Development for Oncology Research & Cancer Immunotherapy Using 3D Biology: Simultaneous Digital Counting of DNA, RNA, and Proteins at 800-plex. Kit Fuhrman, Ph.D., NanoString Technologies, 2/17/16.
- Using Scalable Transfection to Optimize and Simplify Cell-Based Projects. James Brady, Ph.D., MaxCyte, 3/22/16.
- High Resolution In Vivo Micro-Ultrasound & Photoacoustic Imaging. Kelly O'Connell, Ph.D., VisualSonics, 3/15/16.
- Real-Time Quantitative Live-Cell Analysis. Lindy O’Clair, Essen Biosciences, 4/12/16.
- Enabling National-Scale Cancer Genomics on the Cloud. Gaurav Kaushik, Ph.D., SevenBridges, 5/3/16.
- Fluorescence Microscopy: Modern Techniques of High- and Super-Resolution of Biological Specimens. David Hitrys, GE Healthcare Life Sciences, 5/11/16.
- RapidFire System: 10X Faster Detection of Native Compounds by Mass Spectrometry. Michelle Romm, Ph.D., Agilent Technologies, 5/23/16.
- Unveiling the Tumor Microenvironment Using Imaging Mass Cytometry. Raúl Catena, Ph.D., Institute for Molecular Life Sciences, University of Zürich, 6/6/16.
- Next Generation Sequencing Solutions for Immune Profiling, Analysis of Small RNAs, and NGS Sample Preparation.Anthony Kayas, Takada Clontech, 6/23/16.
- How Multiphoton Microscopy Can Help Unveil Cognitive Deficits Associated with Chronic Stress and Depression.Conor Liston, M.D., Ph.D., Brain and Mind Research Institute and Department of Psychiatry, Weill Cornell Medicine, 6/28/16.
- X-Ray Microscopy for the Life Sciences. Ruth Redman, Ph.D., Carl Zeiss Microscopy, 6/30/16.
- High-throughput Gene Expression Profiling of Complex Cell Populations Using the 10X Chromium Single Cell System.Paul Ryvkin, Ph.D., 10X Genomics. 7/14/16.
- Mobile Research Studies: The What, Why, and How. Michael Carroll, Cornell Tech NYC, and Deborah Estrin, Ph.D. Cornell Tech NYC and Weill Cornell Medicine. 7/19/16.
- Molecular Barcoding Approaches for Targeted RNA Sequencing: Digital RNA-seq for Gene Expression Studies. Samuel J. Rulli, Jr., Ph.D., Qiagen Advanced Genomics. 7/21/16.
- Liquid Handling Reimagined: Echo Acoustic Liquid Handling for Screening, Assay Assembly, and Genomics Workflows. Tim Allison, Ph.D., LabCyte. 7/25/16.
- Drop-Sequencing for High-throughput Single Cell Gene Expression Studies. Olivier Elemento, Ph.D. and Asaf Poran, Computational Biomedicine, Weill Cornell Medicine. 8/2/16.
- Flow Cytometry Seminar. Overview of the New CLC Flow Cytometry Core Facility. Jason McCormick and Tomas Baumgartner, Weill Cornell Medicine. Beyond the Cytometer: New Approaches for Multicolor Panel Design and Novel Applications for Flow Cytometry. Arielle Ginsberg, BD Biosciences. 8/18/16.
Tri-I Research Resources Expo
Summary
The Tri-I Research Resources Expo offers a showcase for the life sciences and biomedical core facility resources and services located at all the Tri‐Institutions, including Weill Cornell Medicine (WCM), Weill Cornell Medicine-Qatar (WCM-Q), Cornell University (CU), The Rockefeller University (RU, and Memorial Sloan Kettering Cancer Center (MSKCC). The Expo was held for the first time on June 2 - 3, 2016, sponsored by the Tri‐Institutional Collaboration Network (TCN). The first day of the Expo included an internal roundtable discussion for the core directors, a public seminar about the core resources on all the campuses, and a poster session in New York City, NY, followed the next day by a poster session in Ithaca, NY. The poster sessions on each campus were followed by informal tours of cores.
The internal core director discussion session included live video conference participation from Ithaca and Qatar. The public seminar included on-site presentations by representatives from RU, MSKCC and WCM, and live video presentations by representatives from WCM-Q and Cornell Ithaca. There were a total of 53 core posters, with 39 posters presented at the NYC session and 31 presented at the Ithaca session.
Click here for the Tri-I Research Resources Expo 2016 program.
Expo 2016 Posters
- WCM Core Laboratories Center (CLC)
- Cornell Biotechnology Resource Center (BRC)
- WCM CLC Genomics and Epigenomics Core: Genomics Services
- WCM CLC Genomics and Epigenomics Core: Epigenomics Services
- WCM CLC/MCC Proteomics and Metabolomics Core
- Cornell BRC Proteomics and Mass Spectrometry Facility
- MSKCC Proteomics and Microchemistry Core Facility
- RU Proteomics Resource Center
- WCM CLC NMR Core
- Cornell NMR Core
- WCM CLC Milstein Chemistry Core
- WCM CLC Flow Cytometry Core
- RU Flow Cytometry Resource Center
- WCM CLC Citigroup Biomedical Imaging Center
- Cornell MRI Facility
- WCM CLC Imaging Core
- Cornell BRC Imaging Facility
- Cornell BRC Imaging Facility: High Resolution 3D X-Ray CT
- Cornell BRC Imaging Facility: Flow Cytometry Service
- BTI Pant Cell Imaging Center
- WCM BMRI Neuroanatomy Electron Microscopy Core
- RU Electron Microscopy Resource Center
- WCM Visual Function Core
- RU High Throughput & Spectroscopy Resource Center
- MSKCC RNAi and CRISPR Core
- MSKCC High Content Screening Core
- MSKCC High Throughput Compound Screening Core
- WCM CLC Applied Bioinformatics Core (ABC)
- Cornell BRC Bioinformatics Facility
- Cornell BRC Bioinformatics Facility: BioHPC Computing Laboratory
- Cornell BRC Bio-IT Facility
- WCM Biostatistics and Epidemiology Consulting Service
- Cornell Statistical Consulting Unit (CSCU)
- WCM Scientific Computing Unit
- Cornell Center for Advanced Computing (CAC)
- Cornell Research Data Management Services Group
- WCM Architecture for Research Computing in Health (ARCH)
- CTSC Biomedical Informatics Core
- CTSC Cores
- WCM Belfer Gene Therapy Core Facility
- MSKCC Cell Therapy and Cell Engineering Facility
- Tri-I Stem Cell Derivation Core
- Cornell Stem Cell and Transgenic Core Facility
- Cornell Stem Cell Pathology Unit
- Cornell Plant Transformation Facility
- Cornell Nanobiotechnology Center (NBTC): Capabilities and Techniques
- Cornell Nanobiotechnology Center (NBTC): Materials Characterization
- Cornell Center for Materials Research (CCMR)
- Cornell NanoScale Science and Technology Facility (CNF)
- MSKCC-RU Antibody & Bioresource Core Facility
- MSKCC Antitumor Assessment Core Facility
- Cornell NanoScale Science and Technology Facility (CNF)
- Cornell Center for Advanced Technology (CAT)
CTSC Services and Resources
Support cores and related resources are essential to providing the infrastructure to conduct research. The CTSC provides a multi-institutional collection of resources and service providers to assist researchers throughout the protocol lifecycle, from the creation of interdisciplinary research teams, to protocol design and development, through implementation, compliance, and publication of results.
Visit the CTSC Services and Resources website.
